Published on: March 8, 2025

Click the start button to start the Mocktest
#1. A solid having irregular shape is called_______.
#2. Graphite, diam0oond and fullerene are the polymorphic forms of?
#3. Which metal crystallizes in a simple cubic structure
#4. Which among the followings metal crystallise as a simple cube?
#5. The relation between solubility of a gas in liquid at constant temperature and external pressure is stated by which law?
#6. 9 g of glucose (mol wt = 180) is dissolved in 90 g of H2O. Relative lowering of vapour pressure is _____.
#7. Relative lowering of vapour pressure of a dilute solution of glucose dissolved in 1 kg of water is 0.002. The molality of the solution is ____.
#8. If molality of the dilute solution is doubled, the value of molal depression constant (Kf ) will be ______.
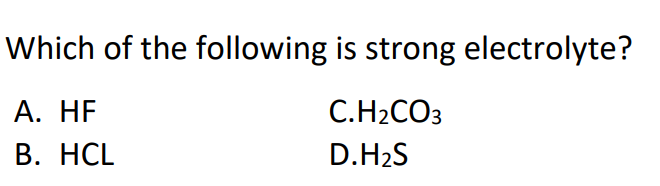
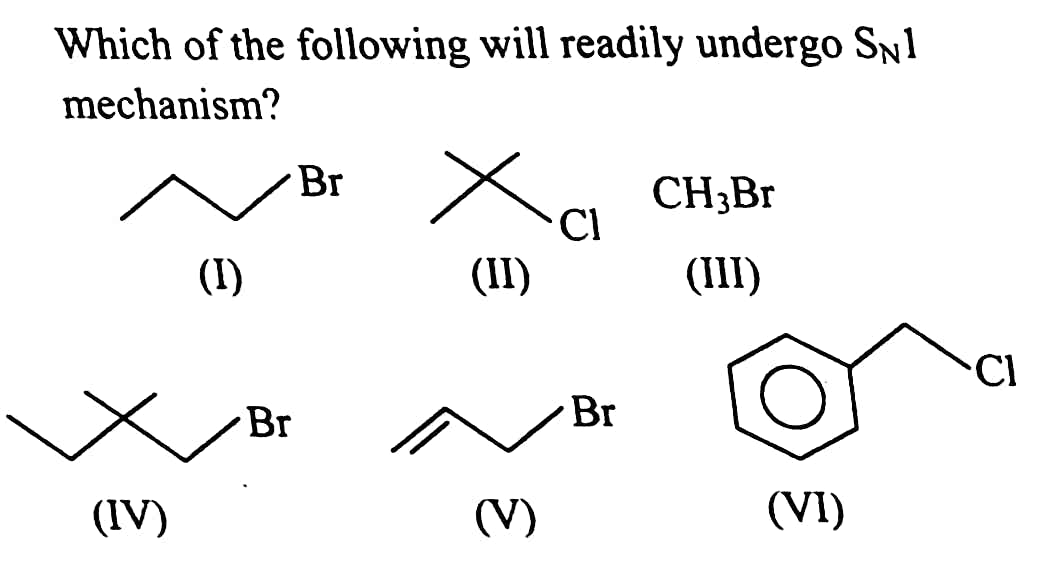
#9.
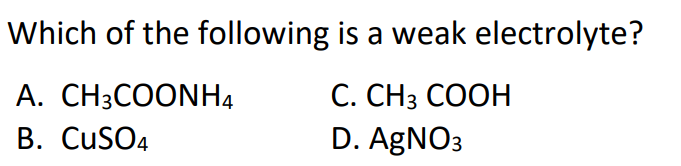
#10.

#11.

#12.
#13. Identify an extensive property among the following
#14. Calculate the work done during compression of 2 mol of an ideal gas from a volume of 1 m cube to 10 dm cube at 300 K against a pressure of 100 kPa.
#15. How internal energy change due to removal of heat and work from the system?
#16. Heat formation of water is -272 kJ. How much water can be decomposed by 750 kJ of heat?

#17.

#18.

#19.
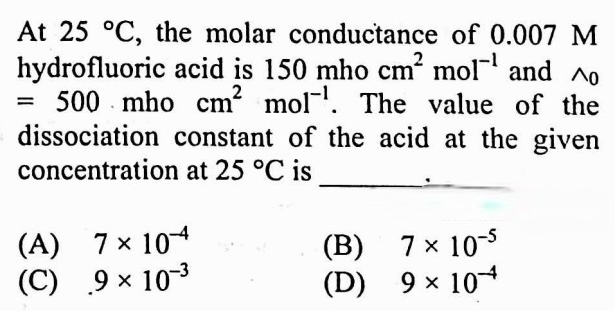
#20.
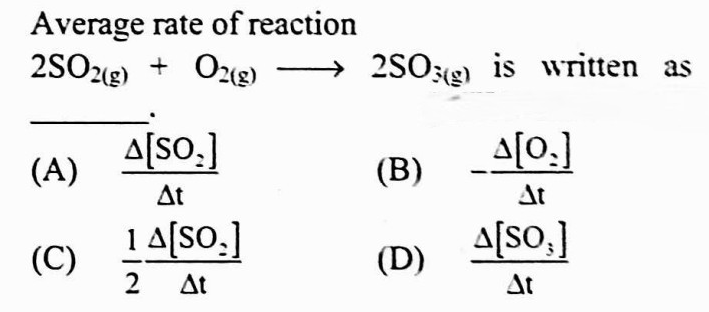
#21.
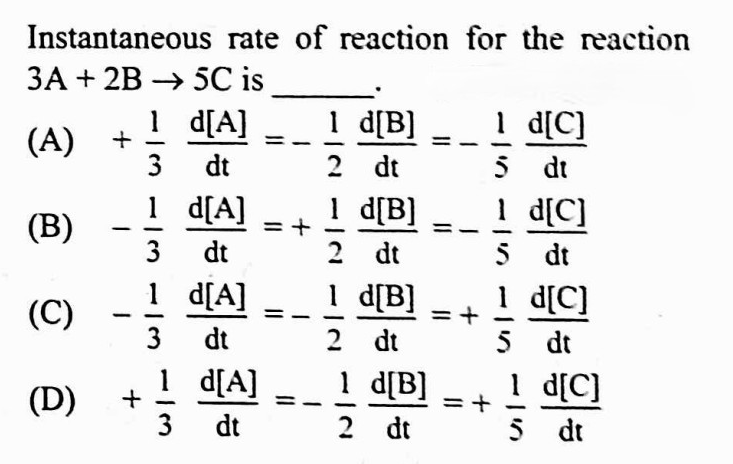
#22.
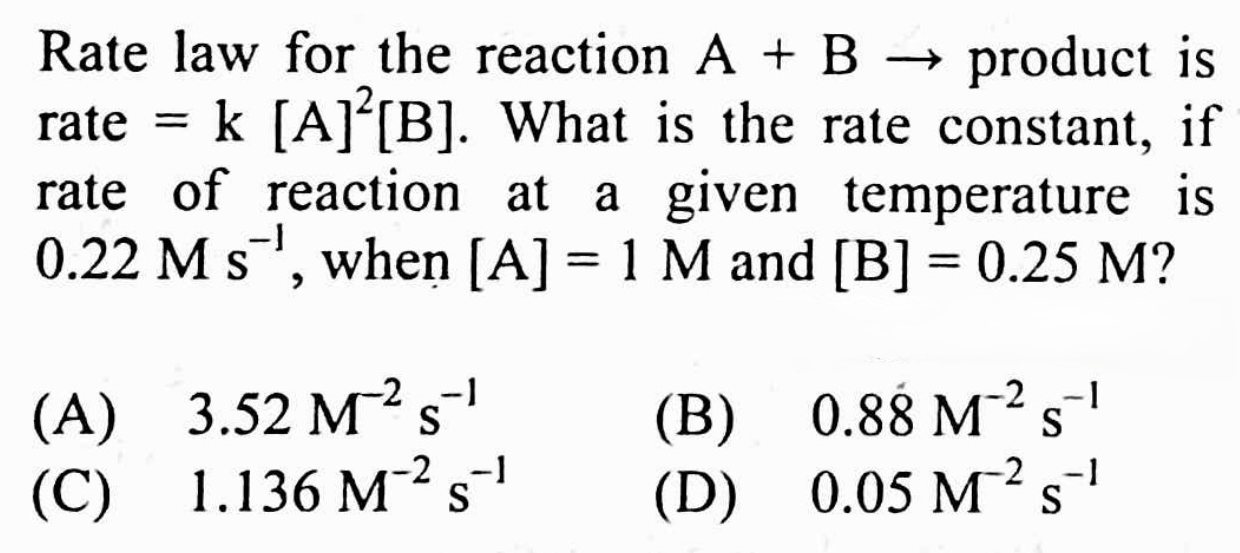
#23.
#24. In a reaction A -> B, if the concentration of reactant is increased by 9 times, then rate of reaction increases 3 times. What is the order of reaction?

#25.
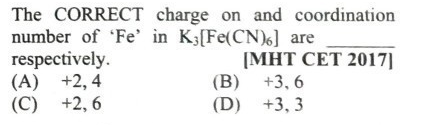
#26.
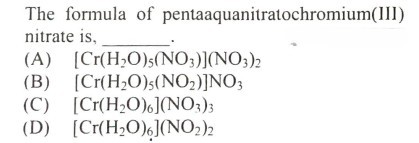
#27.
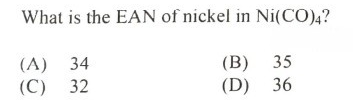
#28.
#29. Which one of the following is a transition element as per the ground state electronic configuration?
#30. Which of the following mineral of iron is in the form of carbonate?
#31. A compound absorbs light in the wavelength region 490-500 nm. Its complementary colour is:
#32. Potassium dichromate is a good oxidizing agent, in acidic medium the oxidation state of chromium changes by______.
#33. Coordination compound contain ligands attached to central metal atom/ion through _____ bonds.
#34. Ammonia molecule and oxalate ion have _____ coordination sites respectively.